Dopamine Agonist Pathological Gambling And Hypersexuality
Frequency of New-Onset Pathologic Compulsive Gambling or Hypersexuality After Drug Treatment of Idiopathic Parkinson Disease
J. Michael Bostwick, MD, Kathleen A. Hecksel, MD, Susanna R. Stevens, MS, James H. Bower, MD, and J. Eric Ahlskog, MD, PhD
+ Author Affiliations
Dopamine agonist treatment of PD carries a substantial risk of pathological behaviors. These occurred in 16% of agonist-treated patients; however, when assessing patients whose dose was at least minimally in the therapeutic range, the frequency jumped to 24%. Pathological gambling and hypersexuality were most common. Impulse control disorder (ICD), including pathological gambling, hypersexuality, and compulsive shopping has been linked to antiparkinsonian medication, especially dopamine agonists. The mechanism of ICD is not completely clear, but it seems that ICD is the result of an activation of dopamine receptors, mostly D3 in the ventral striatum. Patients treated with dopamine agonists that have.
From the Department of Psychiatry and Psychology (J.M.B.), Department of Health Sciences Research (S.R.S.), and Department of Neurology (J.H.B., J.E.A.), Mayo Clinic, Rochester, MN; and Department of Psychiatry, University of Michigan, Ann Arbor (K.A.H.)
Individual reprints of this article are not available. Address correspondence to J. Michael Bostwick, MD, Department of Psychiatry and Psychology, Mayo Clinic, 200 First St SW, Rochester, MN 55905 (bostwick.john@mayo.edu).
Next Section
Abstract
OBJECTIVE: To determine the frequency of new-onset compulsive gambling or hypersexuality among regional patients with Parkinson disease (PD), ascertaining the relationship of these behaviors to PD drug use.
PATIENTS AND METHODS: We retrospectively reviewed the medical records of patients from 7 rural southeastern Minnesota counties who had at least 1 neurology appointment for PD between July 1, 2004, and June 30, 2006. The main outcome measure was compulsive gambling or hypersexuality developing after parkinsonism onset, including the temporal relationship to PD drug use.
RESULTS: Of 267 patients with PD who met the study inclusion criteria, new-onset gambling or hypersexuality was documented in 7 (2.6%). All were among the 66 patients (10.6%) taking a dopamine agonist. Moreover, all 7 (18.4%) were among 38 patients taking therapeutic doses (defined as ≥2 mg of pramipexole or 6 mg of ropinirole daily). Behaviors were clearly pathologic and disabling in 5: 7.6% of all patients taking an agonist and 13.2% of those taking therapeutic doses. Of the 5 patients, 2 had extensive treatment for what was considered a primary psychiatric problem before the agonist connection was recognized.
CONCLUSION: Among the study patients with PD, new-onset compulsive gambling or hypersexuality was documented in 7 (18.4%) of 38 patients taking therapeutic doses of dopamine agonists but was not found among untreated patients, those taking subtherapeutic agonist doses, or those taking carbidopa/levodopa alone. Behaviors abated with discontinuation of agonist therapy or dose reduction. Because this is a retrospective study, cases may have been missed, and hence this study may reflect an underestimation of the true frequency. Physicians who care for patients taking these drugs should recognize the drug’s potential to induce pathologic syndromes that sometimes masquerade as primary psychiatric disease.
Both the medical press and the popular press have recently drawn attention to pathologic compulsive behaviors provoked by drug therapy for Parkinson disease (PD); however, the frequency of these socially devastating behaviors in a community sample of patients with idiopathic PD is unknown. In nearly all cases, pathologic gambling has been linked to dopamine agonist treatment: pramipexole, ropinirole, pergolide, cabergoline, or bromocriptine.1-13 In contrast, only rare cases of pathologic gambling have been associated with carbidopa/levodopa monotherapy.1-12 In most cases, affected patients either had no gambling history or gambled only occasionally for recreation. Pramipexole has been the agonist most often implicated, although this might primarily reflect prescribing practices.11
Pathologic hypersexuality has also been associated with dopamine agonist treatment, and agonists have been implicated in approximately 90% of reported cases; as with pathologic gambling, carbidopa/levodopa monotherapy has rarely been culpable.4,8,10,13-18 Similar to agonist-induced pathologic gambling, hypersexuality has surfaced among people with no history of sexual indiscretions. Other compulsive behaviors have additionally been provoked by dopamine agonist treatment, including compulsive eating,17,19 excessive shopping or spending,4,8,10,17,18 and less socially concerning activities, such as compulsive fishing, compulsive gardening, or excessive engagement in hobbies.20
Pathologic gambling and hypersexuality related to dopamine agonists have often had devastating consequences that may overshadow even the symptoms of PD.2,17 These drugs are routinely advocated as first-line treatment of PD, especially in younger patients. In view of their common use, we wanted to assess the risks of these medication-induced behavioral syndromes. Prevalence reports to date have emanated from referral center specialty clinics. To reduce specialty referral bias, we limited our sample to patients with PD who reside in the largely rural 7-county radius around Mayo Clinic’s site in Rochester, MN.
Previous Section
Next Section
PATIENTS AND METHODS
The Mayo Clinic Health Sciences computer database was queried to identify all patients residing in 7 southeastern Minnesota counties seen in the Mayo Clinic Neurology Department between July 1, 2004, and June 30, 2006, for evaluation or treatment of PD. By limiting the study population to the 7 counties surrounding Mayo Clinic’s site in Rochester, we attempted to constitute a sample of patients who receive their neurologic care locally. We are mindful that residents of these counties could seek care elsewhere but assume that most came to Mayo Clinic, given its proximity and the dearth of alternative care in these largely rural counties. From the medical record review, we included only patients who met the clinical criteria for probable idiopathic PD, as previously defined21-23; these criteria included at least 2 of 4 cardinal signs (resting tremor, bradykinesia, rigidity, and impaired postural reflexes) and excluded drug-induced parkinsonism, other secondary parkinsonisms, and parkinsonism-plus disorders. Cognitive impairment that developed less than 1 year after parkinsonism onset (suggesting dementia with Lewy bodies)24 was an exclusion criterion. Patients who had later-developing dementia (>1 year after parkinsonism) were not excluded.
View this table:
In this window In a new window
TABLE 1.
Primary Symptomatic Medications in 267 Patients With Parkinson Disease
Among patients with PD, we identified the medical records that documented pathologic compulsive gambling or hypersexuality beginning after the onset of parkinsonism, excluding those records in which the behavior predated the parkinsonism onset. In Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) terms, behaviors considered pathologic cause “impairment in social or occupational functioning.”25 We included cases of new-onset hypersexuality or gambling that had not definitely reached pathologic proportions; these cases have previously been termed subsyndromal.8 Newly developing compulsive behaviors other than hypersexuality or gambling were also recorded. Note that case ascertainment was limited by the study’s retrospective nature, and our findings likely underestimate the occurrence of these behaviors.
We additionally tabulated demographic data, psychoactive medications, marital status, psychiatric history, and any other addictive behaviors, including current tobacco or alcohol use or dependence. Dopamine agonists were a focus of this study, and these drugs are always started in low, subtherapeutic doses; often, however, they are inadvertently maintained at a low dose, insufficient for a therapeutic PD effect.26 Thus, we stratified dopamine agonist-treated patients into those taking therapeutic and subtherapeutic doses based on prior criteria26: 2 mg/d or more for pramipexole and 6 mg/d or more for ropinirole. These agents were the only dopamine agonists prescribed for PD in the United States at the time of the current study. Dementia status was recorded with criteria as previously published27; because mild cognitive impairment could not be reliably and consistently identified in this retrospective medical record review, this was not tabulated.
STATISTICAL ANALYSES Data are presented as number and percentage for categorical variables and mean ± SD and median (range) for continuous variables. P values comparing independent groups of patients are from the Fisher exact test for categorical variables and either the Wilcoxon rank sum test for 2-group comparisons or the Kruskal-Wallis test for 3-group comparisons of continuous variables.
Previous Section
Next Section
RESULTS
PATIENT CHARACTERISTICS Study entry criteria were met by 267 patients with PD who were seen at least once by a Mayo Clinic neurologist during the 2-year study period. Of these 267 patients, 66 (24.7%) were taking a dopamine agonist, but only 38 (14.2%) were taking agonist doses in the therapeutic range as defined herein. Of the remaining 201 patients, 178 (88.6%) were taking carbidopa/levodopa without an agonist (Table 1). During this 2-year interval, the 267 patients with PD were seen a mean ± SD of 3.1±2.6 times in the Mayo Clinic Neurology Department.
New-onset hypersexuality or gambling was documented in 7 patients and was clearly pathologic in 5. In the 2 patients in whom this behavior was not clearly pathologic, hypersexuality produced marital strife sufficient to discuss with the physician and sufficient for the physician to document in the medical record. All 7 patients with hypersexuality or gambling were taking a dopamine agonist, either pramipexole or ropinirole; 2 were taking it without carbidopa/levodopa. Furthermore, all 7 were taking therapeutic doses of pramipexole (≥2 mg/d) or ropinirole (≥6 mg/d). Thus, 7 (10.6%) of the 66 patients taking a dopamine agonist in any dose experienced hypersexuality or gambled. Among the 38 patients taking therapeutic doses of a dopamine agonist, 7 (18.4%) experienced hypersexuality or gambled (Figure). Restricting this to only the 5 patients with clearly pathologic gambling or hypersexuality revealed that these problems developed in 7.6% of the 66 agonist-treated patients with PD and 13.2% of 38 patients taking therapeutic doses of a dopamine agonist.
View larger version:
In this page In a new window
Download as PowerPoint Slide
FIGURE.
Percentage of patients with Parkinson disease (PD) with compulsive behaviors, stratified by dopamine agonist dose.
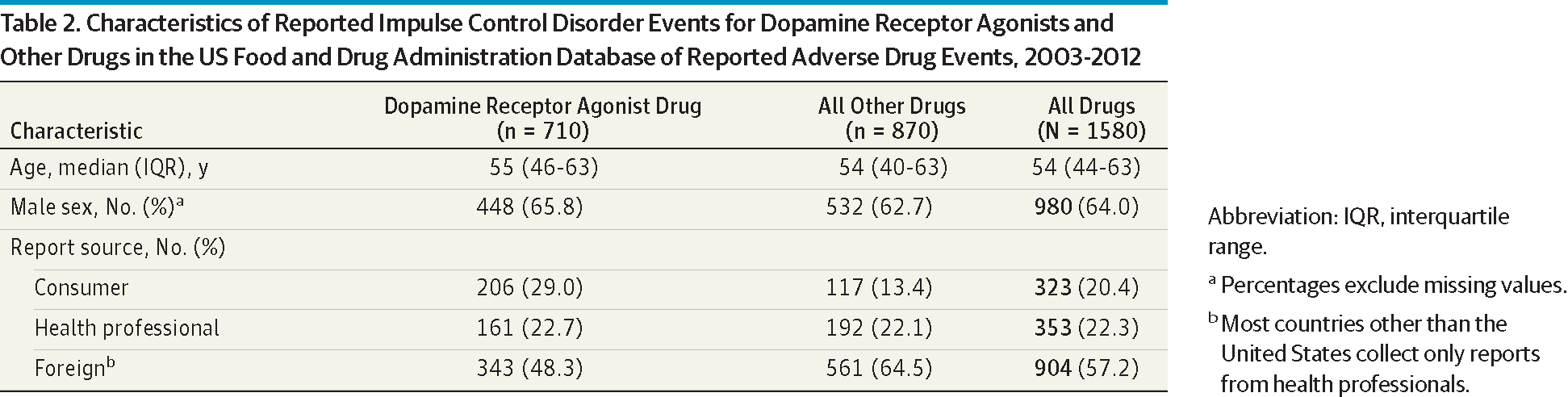
As indicated in Table 2, these 7 patients were significantly younger than the remaining PD patients and were significantly more likely to be smokers. Otherwise, this group was similar to the other patients with PD in a variety of other categories (Table 2). None of the 7 patients had dementia except for patient 5, who had Hoehn-Yahr PD stage 4 disease, whereas the other 6 patients all had stage 2 PD with preserved balance.28,29 Individual characteristics of these 7 patients are given in Table 3. Note that the agonist dosages did not exceed the usual recommended dosage in any patient.
Parenthetically, all 7 of these patients had been seen at least once by a Mayo Clinic staff neurologist who specializes in movement disorders and who had confirmed the diagnosis of PD; however, not all 7 were being followed up by a movement specialist at the time these behaviors had surfaced. Although these patients were independently identified by reviewing the 267 medical records, it was subsequently noted that 3 had previously been described in case series of patients reported from our medical center.2,17,20
View this table:
In this window In a new window
TABLE 2.
Demographics and Characteristics of the 267 Study Patientsa
View this table:
In this window In a new window
TABLE 3.
Patients With PD and Compulsive Gambling or Hypersexualitya
REPORT OF CASES Case 1. After 3 years of parkinsonism, a 46-year-old married man was diagnosed as having PD, and ropinirole monotherapy was initiated, with the dosage gradually increased to 15 mg/d. He subsequently developed “excessive libido” that “occasionally causes arguments with my wife.” Later carbidopa/levodopa was added, and the hypersexuality persisted for the next 2 years, with the patient or wife mentioning this to physicians at least 2 other times. Ultimately, with the hypersexuality “becoming a marital issue,” the dose of ropinirole was tapered and replaced with entacapone, leading to reduction of problematic libido.
Case 2. A 52-year-old married man with an 11-year history of levodopa-responsive PD developed a compulsive gambling habit 5 months after starting pramipexole therapy and 2 months after reaching the maintenance dose of 4.5 mg/d. He had previously gambled at casinos in Las Vegas, NV, only once or twice a year; he never had trouble stopping and never lost large sums of money. Now, he gambled daily, “sometimes [for] 36 hours straight,” sometimes awakening in the middle of the night and driving to the casino. His wife commented that this activity was “completely out of character for him.” His losses totaled $15,000. He also engaged in compulsive lawn care activities, consisting of blowing leaves for up to 6 hours at a time, finding it difficult to stop. Within weeks of stopping pramipexole therapy, the compulsion to gamble abated completely.
Case 3. A 68-year-old man was admitted to the psychiatry department after gambling more than $200,000 in a 6-month period. His family also noted “hypersexuality,” and he harbored the delusion that his ex-wife was a prostitute. His only medications were pramipexole, 4.5 mg/d, and levodopa, 600 mg/d (as carbidopa/levodopa), initially administered when PD was diagnosed 3 years earlier. His daughter said that he had formerly been financially meticulous, never going to casinos and never gambling. During his psychiatric hospitalization, treatment with olanzapine, 7.5 mg/d, plus citalopram, 20 mg/d, was started. Gambling ceased until he elected to stop taking the olanzapine 8 to 9 months later. Compulsive gambling reemerged and persisted for the next 2 years despite multiple psychiatric consultations. It subsequently remitted when his neurologist recognized pramipexole as potentially culpable and discontinued use of the drug.
Case 4. A 53-year-old married woman recently diagnosed as having PD developed uncontrollable urges to gamble within a month of starting pramipexole monotherapy at a maintenance dose of 4.5 mg/d. Unbeknownst to her husband, she lost $50,000 at a local casino during the ensuing 3 years. Before starting pramipexole therapy, she was an indifferent gambler, going to the casino weekly with friends but frequently wagering nothing and never losing large amounts of money. When pramipexole therapy was discontinued and carbidopa/levodopa therapy maintained, the gambling completely abated.
Case 5. An 80-year-old married man with a 16-year history of PD and gradually encroaching dementia developed hypersexuality, with the medical record documenting “some suggestion of sexual inappropriateness” toward his wife. His PD medications at that time were ropinirole, 6 mg/d, and carbidopa/levodopa, 850 mg/d. After the ropinirole dose was tapered, the medical record did not mention hypersexuality again.
Case 6. A 69-year-old married man with a 14-year history of PD reported a 3-year history of gambling and “increased libido.” Prior to 3 years ago, he had never gambled and had gambled only since then during the summer months, “twice a day, every day,” because a casino was close to their summer home. Pramipexole, 4.5 mg/d, had been initiated a year before the onset of these behaviors after he had been taking 3 mg/d for the prior 4 years. After he reduced his pramipexole dose back to 3 mg/d, the problematic gambling remitted.
Case 7. A 49-year-old married man with an 8-year history of PD developed a compulsive gambling habit a month after increasing his ropinirole dosage from 15 to 21 mg/d. Initially regarded as a psychiatric problem, the compulsive gambling was managed with counseling and attendance at Gamblers Anonymous meetings, which controlled the urge but did not eliminate it. A year later, his wife told the neurologist that he had “an excessive sex urge,” later necessitating a call to the police after her husband chased her when she refused sex. Other new-onset compulsions included intense focus on his hobby of making stained glass windows, often staying up all night to work on these. His appetite and alcohol intake also increased. Quetiapine was initiated at dosages up to 300 mg/d without effect on his sexual demands. He was diagnosed as having bipolar disorder, and his medical regimen was switched from quetiapine to carbamazepine without benefit. Not until the dosage of ropinirole (then 24 mg/d) was tapered and carbidopa/levodopa substituted did his pathologic behaviors remit, with his wife reporting a “complete transformation” with the levodopa and entacapone. “I have my husband back,” she said. “It was like I was married to an alien.”
Previous Section
Next Section
DISCUSSION
We assessed the risk of serious pathologic behavioral syndromes that develop with treatment of PD. Restricting the analysis to gambling or hypersexuality that was unequivocally pathologic revealed a frequency of 13.2% among those taking therapeutic doses of pramipexole or ropinirole. The risk increased to 18.4% by including the 2 patients with hypersexuality that produced marital strife that was not clearly pathologic. This finding contrasts with that of no such cases among the 178 patients taking carbidopa/levodopa without an agonist or the 28 taking subtherapeutic agonist doses.
These data are consistent with multiple prior studies reporting that these pathologic behaviors are highly associated with dopamine agonist use.1-13,17,18 The literature indicates that the risk of pathologic gambling or hypersexuality with carbidopa/levodopa monotherapy is low,1-12,17,18 which is also consistent with our findings. However, most of the described patients with PD, including our patients, have been taking an agonist concurrent with levodopa, which presumably facilitates the effect.
These pathologic behaviors have been noted almost exclusively among patients with PD who have been taking dopamine agonist doses well into the therapeutic range or beyond,2,3,11,17,18 consistent with our findings. Dopamine agonists are always initially administered in low doses that are far below therapeutic for PD. The conventional escalation to therapeutic doses for PD requires at least 4 to 6 weeks.26 In routine practice, PD is often inadvertently maintained with low doses, perhaps because of the complexity, multiple steps, and time required for this escalation. Our study and prior investigations suggest that patients with PD who are taking subtherapeutic doses have a low risk of developing these pathologic behaviors (although defeating the purpose of these PD drugs). However, there are exceptions; most notably, gambling or hypersexuality has been reported in a few patients treated with low agonist doses for restless legs syndrome.30-32
Also consistent with other series, patients with PD who are experiencing these problems tend to be younger and usually do not have dementia. However, younger patients are more likely to have agonists prescribed, whereas agonists are less frequently prescribed in PD patients with dementia (treatment bias).
These retrospective findings are conservative risk estimates and should be viewed as an estimate of the minimum frequency for several reasons. First, patients often fail to report these problems to their caregivers (and may not, even if asked, because of embarrassment). Second, physicians may not include such problems in the medical record to avoid socially embarrassing documentation. Third, in this retrospective study, there is no assurance that physicians asked, let alone recorded these data. Finally, if treating physicians are unaware of the potential for such behavior, it is unlikely to be recognized. Thus, these behaviors were not identified in the 2 major long-term clinical trials of ropinirole33 and pramipexole34 that were conducted before the general recognition of this syndrome. These 2 trials collectively included approximately 180 patients with PD who were treated with 1 of these agonists for at least 4 years, ultimately in conjunction with carbidopa/levodopa. If the prevalence of these pathologic behaviors is similar to what we and others have found, then cases should have surfaced in these trials.
Questionnaire assessments of patients with PD followed up by referral center specialty clinics have revealed that approximately 12% of those treated with a dopamine agonist experienced pathologic gambling or hypersexuality.4,8 Although these findings suggested substantial risks, such surveys might raise questions of referral or selection bias. However, our similar findings among regional patients with PD suggest that these figures are probably not overestimated and, given the methodological limitations of our study, that the true frequency of these behaviors is likely underestimated.
Note, however, that our study is not truly a community-based study as epidemiologically defined. Although residence was restricted to the 7-county region surrounding Mayo Clinic, patients could have sought care elsewhere, allowing for possible selection bias.
As illustrated by the case histories, the problems can be life-changing events, with gambling depleting family finances or hypersexuality threatening marriage and reputation. Physicians treating PD with dopamine agonists should obviously warn the patients, spouses, and families of such risks because they may not recognize the relationship to the drug until disastrous consequences have occurred. As in most prior reports, these pathologic behaviors often develop de novo in people with no prior histories to suggest such risks. Families are typically stunned by the out-of-character behavior but do not usually link it with the dopamine agonist.
Psychiatrists may be unaware of the potential of dopamine agonists to induce these behavioral syndromes. This was evident in the 2 patients who were receiving extended psychiatric care with multiple therapeutic modalities that proved insufficient until use of the agonist was discontinued. Ultimately, discontinuation of use of the dopamine agonist was consistently effective in treating these patients. The reversibility with discontinued use of the agonist noted herein is consistent with the findings of other recent series of similar patients.2,10,17,18
The basis for these drug-induced pathologic behaviors continues to be debated. However, it is hard to ignore the fact that the implicated dopamine agonists have unique and selective affinity for dopamine D3 receptors.35 Dopamine D3 receptors are primarily confined to the limbic system.36 Conceptually, they may prime the dopamine reward circuits to facilitate certain behaviors. Hence, patients in the current and other series often display more than 1 pathologic behavior.
If selective dopamine D3 agonism mediates these behaviors, one might speculate that selective dopamine D3-blocking drugs might effectively treat such pathologic behaviors that develop spontaneously in the general community. Such drugs exist37 but have not been sufficiently developed to be used in clinical trials.
Previous Section
Next Section
CONCLUSION
Among the study patients with PD, new-onset compulsive gambling or hypersexuality was documented in 7 (18.4%) of 38 patients taking therapeutic doses of dopamine agonists, but was not found among untreated patients, those taking subtherapeutic agonist doses, or those taking carbidopa/levodopa alone. Behaviors were clearly pathologic and disabling in 5 of the 7 patients. The behaviors abated with discontinuation of agonist therapy or dose reduction. Because this was a retrospective study, cases may have been overlooked, and thus this study may reflect an underestimate of the true frequency. Physicians who care for patients taking these drugs should recognize the potential of the drugs to induce pathologic syndromes that sometimes masquerade as primary psychiatric disease.
© 2009 Mayo Foundation for Medical Education and Research
Previous Section
REFERENCES
↵ Driver-Dunckley E, Samanta J, Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology. 2003;61(3):422-423. FREE Full Text
↵ Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol. 2005 Sep;62(9):1377-1381. Epub 2005 Jul 11. Abstract/FREE Full Text
↵ Grosset KA, Macphee G, Pal G, et al. Problematic gambling on dopamine agonists: not such a rarity. Mov Disord. 2006;21(12):2206-2208. CrossRefMedline
↵ Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006;67(7):1258-1261. Abstract/FREE Full Text
Lu C, Bharmal A, Suchowersky O. Gambling and Parkinson disease [letter]. Arch Neurol. 2006;63(2):298. FREE Full Text
Avanzi M, Baratti M, Cabrini S, Uber E, Brighetti G, Bonfà F. Prevalence of pathological gambling in patients with Parkinson’s disease. Mov Disord. 2006;21(12):2068-2072. CrossRefMedline
Szarfman A, Doraiswamy PM, Tonning JM, Levine JG. Association between pathologic gambling and Parkinsonian therapy as detected in the Food and Drug Administration Adverse Event Database [letter]. Arch Neurol. 2006;63(2):299-300. FREE Full Text
↵ Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006 Oct;67(7):1254-1257. Epub 2006 Sep 6. Abstract/FREE Full Text
Voon V, Hassan K, Zurowski M, et al. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006;66(11):1750-1752. Abstract/FREE Full Text
↵ Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63(7):969-973. Abstract/FREE Full Text
↵ Gallagher DA, O’Sullivan SS, Evans AH, Lees AJ, Schrag A. Pathological gambling in Parkinson’s disease: risk factors and differences from dopamine dysregulation: an analysis of published case series. Mov Disord. 2007;22(12):1757-1763. CrossRefMedline
↵ Voon V, Thomsen T, Miyasaki JM, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007;64(2):212-216. Abstract/FREE Full Text
↵ Singh A, Kandimala G, Dewey RB Jr., O’Suilleabhain P. Risk factors for pathologic gambling and other compulsions among Parkinson’s disease patients taking dopamine agonists. J Clin Neurosci. 2007 Dec;14(12):1178-1181. Epub 2007 Aug 27. CrossRefMedline
Vogel HP, Schiffter R. Hypersexuality—a complication of dopaminergic therapy in Parkinson’s disease. Pharmacopsychiatria. 1983;16(4):107-110. Medline
Uitti RJ, Tanner CM, Rajput AH, Goetz CG, Klawans HL, Thiessen B. Hypersexuality with antiparkinsonian therapy. Clin Neuropharmacol. 1989;12(5):375-383. Medline
Jiménez-Jiménez FJ, Sayed Y, García-Soldevilla MA, Barcenilla B. Possible zoophilia associated with dopaminergic therapy in Parkinson disease. Ann Pharmacother. 2002;36(7-8):1178-1179. Abstract
↵ Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. Pathological hypersexuality predominantly linked to adjuvant dopamineagonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord. 2005;11(6):381-386. CrossRefMedline
↵ Mamikonyan E, Siderowf AD, Duda JE, et al. Long-term follow-up of impulse control disorders in Parkinson’s disease. Mov Disord. 2008;23(1):75-80. CrossRefMedline
↵ Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21(4):524-529. CrossRefMedline
↵ McKeon A, Josephs KA, Klos KJ, et al. Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord. 2007 Dec;13(8):516-519. CrossRefMedline
↵ Stamey W, Jankovic J. Impulse control disorders and pathological gambling in patients with Parkinson disease. Neurologist. 2008;14(2):89-99. CrossRefMedline
Wolters EC, van der Werf YD, van den Heuvel OA. Parkinson’s disease-related disorders in the impulsive-compulsive spectrum. J Neurol. 2008;255(suppl 5):48-56. Medline
↵ Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology. 1999;52(6):1214-1220. Abstract/FREE Full Text
↵ Lippa CF, Duda JE, Grossman M, et al., DLB/PDD Working Group. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812-819. Abstract/FREE Full Text
↵ American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
↵ Ahlskog JE. The Parkinson’s Disease Treatment Book: Partnering With Your Doctor to Get the Most From Your Medications. New York, NY: Oxford University Press; 2005.
↵ Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc. 2003;78(10):1290-1308. Abstract/FREE Full Text
↵ Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427-442. FREE Full Text
↵ Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. CrossRefMedline
↵ Driver-Dunckley ED, Noble BN, Hentz JG, et al. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol. 2007;30(5):249-255. CrossRefMedline
Quickfall J, Suchowersky O. Pathological gambling associated with dopamine agonist use in restless legs syndrome. Parkinsonism Relat Disord. 2007 Dec;13(8):535-536. Epub 2007 Jan 30. CrossRefMedline
↵ Tippmann-Peikert M, Park JG, Boeve BF, Shepard JW, Silber MH. Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology. 2007;68(4):301-303. Abstract/FREE Full Text
↵ Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE, 056 Study Group. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med. 2000;342(20):1484-1491. CrossRefMedline
↵ Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial [published correction appears in Arch Neurol. 2005;62(3):430]. Arch Neurol. 2004;61(7):1044-1053. Abstract/FREE Full Text
↵ Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, Riederer P. Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J Neural Transm. 2003;110(10):1119-1127. CrossRefMedline
↵ Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347(6289):146-151. CrossRefMedline
↵ Joyce JN, Gurevich EV. D3 receptors and the actions of neuroleptics in the ventral striatopallidal system of schizophrenics. Ann N Y Acad Sci. 1999;877:595-613. CrossRefMedline
Articles citing this article
Impact of Dopamine Agonists on Compulsive Behaviors: A Case Series of Pramipexole-Induced Pathological Gambling
B. P. Kolla, M. P. Mansukhani, R. Barraza, and J. M. Bostwick
Psychosomatics May 1, 2010 51(3):271-273
AbstractFull TextPDF
Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation
S. Thobois, C. Ardouin, E. Lhommee, H. Klinger, C. Lagrange, J. Xie, V. Fraix, M. C. Coelho Braga, R. Hassani, A. Kistner, A. Juphard, E. Seigneuret, S. Chabardes, P. Mertens, G. Polo, A. Reilhac, N. Costes, D. LeBars, M. Savasta, L. Tremblay, J. L. Quesada, J. L. Bosson, A. L. Benabid, E. Broussolle, P. Pollak, and P. Krack
Brain April 1, 2010 133(4):1111-1127
AbstractFull TextPDF
Drug-Induced Compulsive Behaviors: Exceptions to the Rule
N. A. Graham, C. J. Hammond, and M. S. Gold
Mayo Clin Proc. September 1, 2009 84(9):846-847
Full TextPDF
This week we have a blog from Dr Carinne Piekema covering the potential role of dopamine agonists in the development of pathological gambling. Carinne is a science journalist and neuroscientist who has worked on executive function and memory in neuroscience laboratories in the Netherlands and the UK. She is currently finishing a Masters in Science Media Production at Imperial College London and is particularly interested in communicating science through radio and podcasting.
Dopamine agonists have been used to alleviate the motor symptoms in Parkinson’s disease (PD) for several decades, both alongside levodopa and – as evidenced by the large number of publications, particularly in the JNNP – as a treatment in its own right (e.g. Parkes et al., 1976; Lees & Stern 1981; Montastruc et al., 1989).
Over the past decade, there have been increasing numbers of reports describing problematic side effects of such dopamine agonists (e.g. Seedat et al., 2000). In particular, a growing body of research shows that dopamine agonist medication may cause pathological gambling, compulsive and impulsive shopping, compulsive eating and hypersexuality in approximately 13.6% of PD patients who receive this treatment, thus developing the clinical symptoms of Impulse Control Disorders (ICD).
While such risk-taking and impulsive behaviours have been extensively documented, the reason why they arise in a particular subgroup of patients is still not fully understood. This is perhaps not surprising when considering the complexity of decision-making processes that might give rise to such risk-taking, impulsive behaviours. Before we choose one option over another, we weigh up the costs and benefits of all the options and calculate the likelihood of positive and negative consequences of our potential choice based on our past experience, and dopamine agonists may bias any of these processes.
I was therefore interested to read two papers published in the last few months that have started to shed light on the causes of the increase in risky decisions in these patients (Claassen et al., 2011; Voon et al., 2011). In both studies, PD patients were tested on varieties of risk-based tasks, both on- and off-dopamine agonist medication, in order to probe, in a controlled manner, the aspects of choice behaviour that might be affected by this type of treatment.
Daniel Claassen and his international team reported in Behavioral Neuroscience how PD patients with and without ICD performed on a paradigm called the Balloon Analogue Risk Task. In this task, participants watch a balloon on a computer screen incrementally filling with air; with every puff of air the value of the balloon is increased by a small amount of money, which they can gain at any time by choosing to pop the balloon. The catch is that, if the balloon pops by accident, all the money in the balloon is lost. Therefore, the participants have to weigh up the cost of an accidental pop against the potential gain from a fuller balloon.
Claasen found that both groups of PD patients, while off-medication, showed a similar pattern of behaviour concerning when to pop the balloon, suggesting that there was no difference in risk-taking behaviour between both groups when they were not taking dopamine agonists. However, when taking dopamine agonist medication, the ICD patients showed an increased tendency compared to those patients without ICD to try to gain more money by letting the balloon inflate further. Moreover, across all PD patients, those who were on the highest doses of dopamine agonist medication were more likely to engage in risky behaviour compared to those who were taking a relatively low dose. Importantly, the researchers found no evidence that ICD patients had problems adjusting their behaviour following negative outcomes, suggesting that these patients are able to respond appropriately to feedback. Thus, it seems that ICD patients’ risk taking is not caused by an inability to learn from mistakes, but instead may result from changes in the way in which each decision is weighed up.

A similar conclusion was reached in a study published in Brain by Valerie Voon and her colleagues in the UK and US. They used a classic gambling task where participants chose between a probabilistic “risky” option and a certain “safe” option. In one condition, the participants were gambling over gains in money (i.e., a choice between a certain small reward or a risky option which could either lead to a high monetary reward or nothing at all) and in the other condition over losses in money (a choice between a certain small loss of money or a risky option which could either lead to a no loss or a large loss of money). In agreement with Claassen and colleagues, the researchers observed that PD patients with ICD who were taking dopamine agonist medication took riskier gambles in the ‘gains’ compared to PD patients without ICD. Nonetheless, these patients with ICD were also still sensitive to the relative level of the risk and chose to gamble less when gambles were more risky and adjusted their behaviour on the trials directly after a loss.
Voon and colleagues also obtained functional neuroimaging data from the patients while they were performing the task in order to look at the underlying neurobiological substrates of these changes in behaviour. They found that PD patients with ICD showed significantly less activity in orbitofrontal and anterior cingulate cortices compared to those without ICD. These regions are known to be involved in the evaluation of risks and value of option and a decreased activity may result in a tendency to behave more riskily.
PD has long been thought of primarily as a disease of the motor system caused by the loss of dopamine-containing cells of the substantia nigra. Such findings have resulted in the idea that the nigrostriatal dopamine system is primarily involved in the selection and control of voluntary movement. However, largely separate from this research, other scientists have been studying another dopamine system – the mesocorticolimbic dopamine system – and have focused mainly on its role in motivational and reward processing. These two strands of dopamine research have largely proceeded in parallel, partly based on an idea that these two dopamine systems were completely distinct (Wise, 2009).
However, these changes in decision-making in subgroups of PD patients documented by Voon, Claassen and other researchers demonstrate that considering PD treatment from the sole perspective of trying to re-equilibrate the loss of nigrostriatal dopamine may have drawbacks. First, given that PD initially primarily affects the nigrostriatal dopamine system while sparing mesocorticolimbic dopamine, dopamine agonists might in fact impair the functionality of the latter system in certain patients, leading to maladaptive decision-making. It may be that PD patients who develop ICD may have underlying genetic neurobiological vulnerabilities that exacerbate such an impairment, similar to the link between impulsive genetic personality traits and drug taking (Dalley et al., 2011).
Second, several lines of basic research suggest that the different dopamine systems interact both anatomically and functionally. Both systems send projections to parts of the frontal lobe as well as the dorsal striatum (e.g. Fallon, 1988), and the cell bodies in both systems can sometimes be found in the same midbrain structure (Wise, 2009). There is also increasing evidence that interactions between ventral striatum – innervated by mesolimbic dopamine – and dorsolateral striatum – innervated primarily by nigrostriatal dopamine – underlie the development of habitual addictive behaviours, and that these interactions are mediated by dopamine transmission (Belin & Everitt, 2008).
But possibly the most compelling reason for why the strong distinction between “motor” and “motivational” dopamine systems may be regarded as artificial can be found in the fact that movement and motivation can not always be so neatly separated: sometimes we choose to walk to the fridge because we are hungry, to scratch our back to relief an itch, to exercise to stay fit. In a study published a few years ago in the Journal of Neuroscience, Pietro Mazzoni and his colleagues showed that one of the key “motor” symptoms of PD – namely bradykinesia – might arise not as the patients could not make the movements, but because they were less motivated to expend energy on movement (Mazzoni et al., 2007). This indicates that nigrostriatal dopamine activity plays a role in enabling voluntary movement not just through modulations of motor pathways in the basal ganglia, but also by participating in calculations of the cost/benefit value of a response. In other words, there is an important class of voluntary movements, which are exactly the type that are frequently affected in PD and rely on dopamine, that are motivationally driven.
Studies such as those led by Claassen and Voon are gradually starting to reveal the mechanisms behind pathological gambling and other compulsive behaviours caused by dopamine agonist medication in PD patients with ICD, and in doing so, they aid our understanding of the role of dopamine in risk-taking behaviour. Even as researchers are trying to uncover the exact functioning of such complex systems, it is important for clinicians to use the knowledge that certain interactions between medication and underlying vulnerabilities can lead to highly undesirable side effects.
Bibliography
Claassen DO, van den Wildenberg WP, Ridderinkhof KR, Jessup CK, Harrison MB, Wooten GF, Wylie SA (2011). The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behav Neurosci DOI: 10.1073/a0023795
Voon V, Gao J, Brezing C, Symmonds M, Ekanayake V, Fernandez H, Dolan RJ, Hallett M. (2011). Dopamine agonists and risk: impulse control disorders in Parkinson’s disease. Brain 134: 1438-1446.
————————————————————————————————————————————
Belin D, Everitt BJ (2008). Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57: 432-441.
Dalley JW, Everitt BJ, Robbins TW (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron 69: 680-694.
Fallon JH (1988). Topographic organization of ascending dopaminergic projections. Ann N Y Acad Sci 537: 1-9.
Lees AJ, Stern GM (1981). Sustained bromocriptine therapy in previously untreated patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 44: 1020-1023.
Mazzoni P, Hristova A, Krakauer JW (2007). Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J Neurosci 27:7105-7116.
Dopamine Hypersexuality
Montastruc JL, Rascol O, Rascol A (1989). A randomised controlled study of bromocriptine versus levodopa in previously untreated Parkinsonian patients: a 3 year follow-up. J Neurol Neurosurg Psychiatry 52:773-775.
Parkes JD, Debono AG, Marsden CD (1976). Bromocriptine in Parkinsonism: long-term treatment, dose response, and comparison with levodopa. J Neurol Neurosurg Psychiatry 39: 1101-1108.
Seedat S, Kesler S, Niehaus DJ, Stein DJ (2000). Pathological gambling behaviour: emergence secondary to treatment of Parkinson’s disease with dopaminergic agents. Depress Anxiety 11: 185-186.
Dopamine And Hypersexuality
Wise RA (2009). Roles for nigrostriatal – not just mesocorticolimbic – dopamine in reward and addiction. Trends Neurosci 32: 517-524.